ARA First in Central Texas to Offer SpineJack Treatment
One of ARA Diagnostic Imaging’s neurointerventional surgeons, Dr. Kirk Conrad, performed the first implantation in Central Texas of the leading-edge SpineJack system on an ARA patient at an Austin hospital. This innovative device helps to restore the natural human anatomy by resetting damaged vertebrae to their original shape and height in a minimally invasive outpatient procedure that takes less than 30 minutes. The implantation of this device provides vertebral body height restoration and pain relief to patients, going beyond traditional methods which focus on pain management.
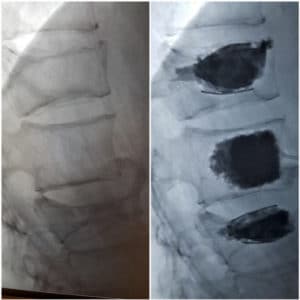
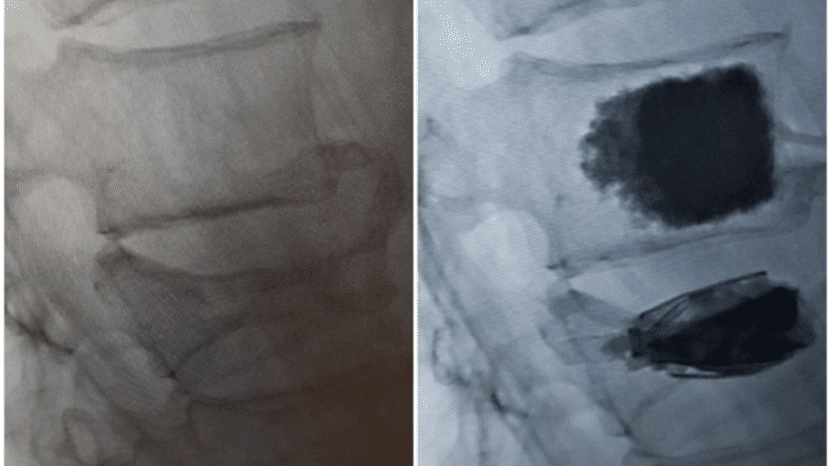
SpineJack devices inserted during kyphoplasty increase vertebral height and provide scaffolding for bone cement filler. (Top and bottom vertebrae)
Osteoporosis and the development of vertebral compression fractures (VCFs)
Implantation of the device represents a significant advancement in treating patients suffering from acute osteoporotic vertebral compression fractures (VCFs). These compression fractures are a common medical condition experienced by patients with a history of osteoporosis (low bone mineral density), metastatic tumors, or those who have experienced trauma to the spine.
VCFs occur when bone tissue in the vertebral body of the spine collapses, causing severe pain, spinal deformity, and loss of height. This condition is commonly caused by osteoporosis, a bone disease characterized by loss of bone mass over time, which results in bones becoming fragile. Often patients experience a continued “downward spiral” since one fracture can lead to another. It is very important that vertebral compression fractures be diagnosed and treated early to stop the progression.
Compression fractures are a serious and growing problem for older adults. In fact, 50% of women and 25% of men will have an osteoporotic fracture in their lifetime.1 Compression fractures of the spine are the most common, with an estimated 700,000 such fractures reported nationally each year.2 Compression fractures can steadily increase with advancing age and have a substantial and negative impact on the quality of life and day-to-day function of those suffering. ARA offers bone density screenings for at-risk people in order to diagnose osteoporosis so it can be medically treated before fractures occur.
Vertebral Compression Fracture—Symptoms and Treatment
Occasionally, patients are unaware that they have a fractured vertebra because they can occur with no pain. Typically, however, compression fractures are followed by a sharp pain and are indicated by one or more of the following symptoms:
- Sudden onset of back pain
- Back pain that worsens while standing or walking
- Limited spinal mobility
- Height loss, deformity, and/or disability
- Kyphosis (commonly referred to as dowager’s hump)
- Crowding of internal organs
Prior to vertebral augmentation, compression fractures were treated by pain management, bed rest, and bracing (limiting mobility). Vertebroplasty and kyphoplasty techniques, introduced in the 1980s and 1990s, gave physicians the option of augmenting the vertebral body with bone cement filler, which stabilized and expanded the vertebra and brought life-changing relief to patients.

SpineJack system application followed by the addition of bone cement filler
SpineJack is a significant improvement to existing treatments and is used in conjunction with bone cement to relieve pain and stabilize the fracture. Because of its extremely stable structure, the SpineJack device acts as a scaffold inside the vertebra and is a step forward in achieving height restoration.
The SpineJack procedure at ARA
The SpineJack application is performed as an outpatient procedure at local Austin hospitals. The procedure is suggested for patients who have been diagnosed with a compression fractures due to osteoporosis that has occurred within the last 4 to 6 months.
Recently cleared by the FDA in the United States, SpineJack is produced domestically by Stryker Instruments and has been used in Europe since 2008 with more than 70,000 units implanted worldwide.
References
- National Osteoporosis Foundation. “Fast Facts on Osteoporosis.” www.nof.org/osteoporosis/diseasefacts.htm. Accessed November 9, 2009.
- Melton LJ 3d. Epidemiology of spinal osteoporosis. Spine 1997 Dec 15;22(24 Suppl):2S-11S.
 Back to Top
Back to Top