Targeted Radiopharmaceutical Therapy for Neuroendocrine Tumors
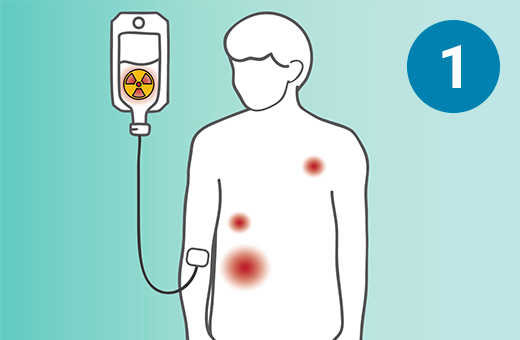
Somatostatin receptor (SSTR)-targeted radiopharmaceutical therapy is an FDA-approved treatment for gastroenteropancreatic neuroendocrine tumors (GEP-NETs). GEP-NETs are tumors that typically arise in the stomach, pancreas, or bowel. They can be cancerous or noncancerous and produce debilitating symptoms. In SSTR-targeted therapy, a radiopharmaceutical is infused into the bloodstream through an IV. It seeks out and attaches to the SSTR protein on neuroendocrine tumor cells wherever they might be located throughout the body. It then delivers a microscopic amount of radiation directly to the neuroendocrine tumor cells, killing them with minimal harm to surrounding healthy tissue. The full treatment is given as four separate infusions, eight weeks apart. During the treatment, you will remain under the care of your cancer care provider, and you will come to the ARA Theranostics Center every eight weeks for your infusion.
SSTR-targeted therapy may help patients with GEP-NETs obtain relief from their symptoms, including skin flushing, diarrhea, wheezing, and rapid heart rate. It can also slow the spread of the tumors, and improve the survival of patients.
The brand name of the SSTR-targeted therapy currently being manufactured and used at the Theranostics Center is Lutathera.
How Does SSTR-targeted therapy work?
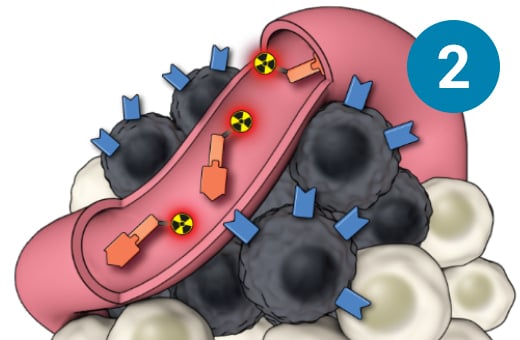
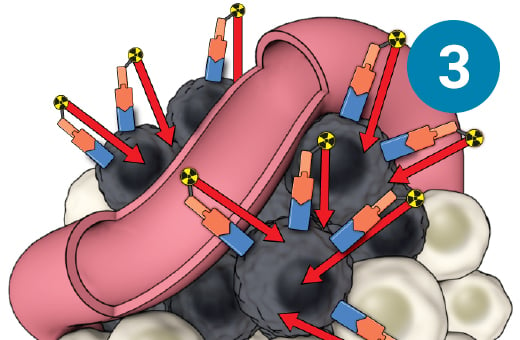
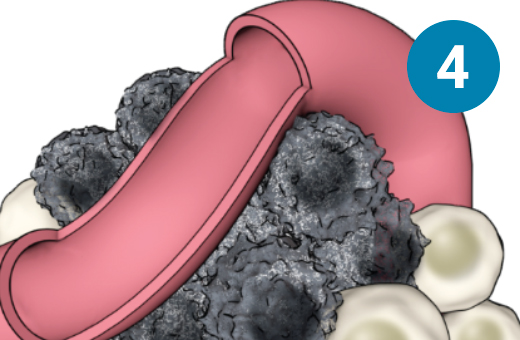
1) SSTR-targeted therapy is infused through an IV. 2) It seeks out diseased cells (in black) anywhere they have settled in the body. 3) It attaches to the diseased cells, and delivers a microscopic amount of radiation to the cells. 4) The radiation kills the diseased cells, leaving surrounding healthy tissue (in white) unharmed.