Targeted Radiopharmaceutical Therapy for Prostate Cancer
Prostate-specific membrane antigen (PSMA)- targeted radiopharmaceutical therapy is an FDA-approved treatment for castrate-resistant metastatic prostate cancer.
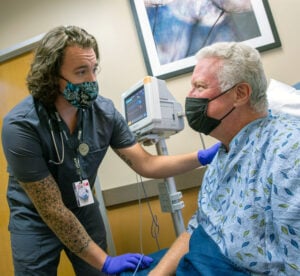
The full treatment is given as six separate infusions, six weeks apart. During the treatment, you will remain under the care of your primary cancer care provider, and you will come to the ARA Theranostics Center every six weeks for your infusion.
PSMA-targeted therapy may be life-extending for patients with metastatic prostate cancer.
To qualify for the PSMA-targeted treatment under current guidelines, patients will need to have been treated with
- Androgen axis inhibition therapy, and
- At least one taxane-based chemotherapy treatment.
If you are interested in finding out if you are qualified for the treatment, please fill out our PSMA Patient Intake Form.
How Does PSMA-targeted Therapy Work?
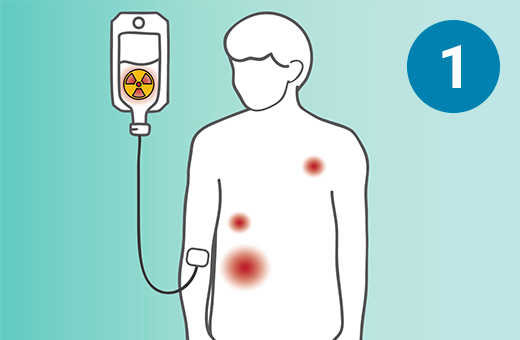
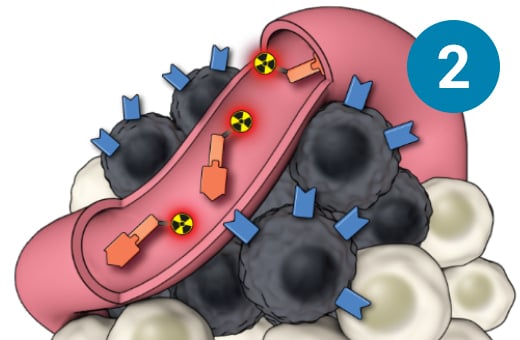
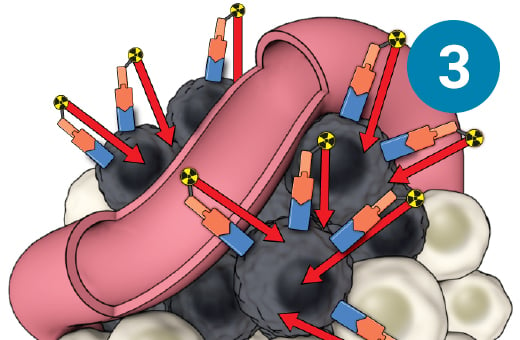
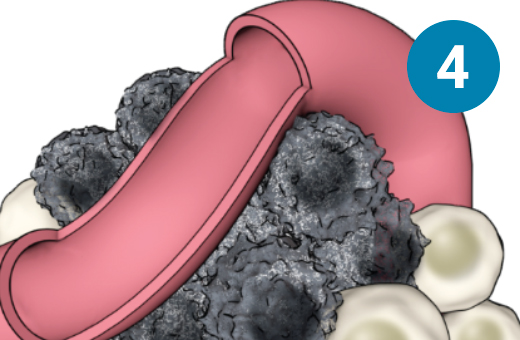
1) The PSMA-targeted radiopharmaceutical, which contains lutetium-177, is infused through an IV. 2) It seeks out prostate cells (in black) anywhere they have metastasized in the body. 3) It attaches to the prostate cancers and delivers a microscopic amount of radiation to the cancer cells. 4) The radiation kills the cancer cells, leaving surrounding healthy tissue (in white) unharmed.